Warning: Undefined array key "file" in /home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/1198/header.php on line 7
Warning: Undefined array key "title" in /home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/1198/header.php on line 7
Warning: Undefined array key "title" in /home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/1198/header.php on line 7
- 非洲人
- 阿爾巴尼亞語
- 阿姆哈拉語
- 阿拉伯
- 亞美尼亞語
- 亞塞拜然語
- 巴斯克
- 白俄羅斯語
- 孟加拉
- 波士尼亞語
- 保加利亞語
- 加泰隆尼亞語
- 宿霧語
- 中國
- 中國(台灣)
- 科西嘉島
- 克羅埃西亞語
- 捷克語
- 丹麥語
- 荷蘭語
- 英語
- 世界語
- 愛沙尼亞語
- 芬蘭
- 法語
- 弗里斯蘭語
- 加利西亞語
- 喬治亞語
- 德文
- 希臘文
- 古吉拉特語
- 海地克里奧爾語
- 豪薩
- 夏威夷人
- 希伯來文
- 沒有
- 苗族
- 匈牙利
- 冰島的
- 伊博語
- 印尼
- 愛爾蘭語的
- 義大利語
- 日本人
- 爪哇語
- 卡納達語
- 哈薩克語
- 高棉語
- 盧安達
- 韓國人
- 庫德
- 吉爾吉斯斯坦
- 結核病
- 拉丁
- 拉脫維亞語
- 立陶宛語
- 盧森堡語
- 馬其頓語
- 馬爾加什
- 馬來語
- 馬拉雅拉姆語
- 馬爾他語
- 毛利人
- 馬拉地語
- 蒙
- 緬甸
- 尼泊爾語
- 挪威
- 挪威
- 奧克語
- 普什圖語
- 波斯語
- 拋光
- 葡萄牙語
- 旁遮普語
- 羅馬尼亞語
- 俄文
- 薩摩亞人
- 蘇格蘭蓋爾語
- 塞爾維亞
- 英語
- 紹納語
- 信德語
- 僧伽羅語
- 斯洛伐克語
- 斯洛維尼亞語
- 索馬利亞
- 西班牙語
- 巽他語
- 斯瓦希里語
- 瑞典
- 他加祿語
- 塔吉克
- 泰米爾語
- 韃靼人
- 泰盧固語
- 泰國
- 土耳其
- 土庫曼語
- 烏克蘭
- 烏爾都語
- 維吾爾
- 烏茲別克語
- 越南語
- 威爾斯語
- 幫助
- 意第緒語
- 約魯巴語
- 祖魯語
Calcium chloride (chemical formula: CaCl2) is a white or yellowish solid inorganic compound, belonging to the salt class, is a typical ionic halide, because of its high solubility, moisture absorption and water removal and widely used in many fields. According to its hydrated form exists in different physical forms, the most common is dihydrate (CaCl2·2H2O), its high solubility allows it to dissolve quickly in water, releasing a large amount of heat, and is therefore very useful in applications requiring rapid heating or drying. In addition, calcium chloride is also commonly used in salt water, including road deicing agents and desiccant used in refrigeration equipment.




Colorless cubic crystal, white or off-white, granular, honeycomb block, spheroid, irregular granular, powdered. Slightly toxic, odorless, slightly bitter taste. It deliqueses easily when exposed to air. It is easily soluble in water, with a solubility of 74.0g /100 g water at 20 ° C, while releasing a large amount of heat (the dissolution enthalpy of calcium chloride is -176.2cal /g). This property makes it very useful in applications where rapid heat generation is required, such as as a heat source in certain heating processes, in addition, the specific heat capacity and thermal conductivity of calcium chloride are also important physical parameters that affect its efficiency in thermal management and regulatory applications. The calcium chloride solution is neutral. Soluble in a variety of polar, protic solvents, solubility at 20℃ in the following solvents (g/100mL solvent) : methanol: 29.2, anhydrous ethanol: 25.8, n-propanol: 15.8, n-butanol: 25.0, n-amyl alcohol: 11.5, ethylene glycol: 21.6 (25℃), formic acid: 43.1, acetic acid: 15.0 (30℃), hydrazine: 16.0. However, in dipolar solvents and low polar solvents, such as ether, tetrahydrofuran, etc., it is only slightly soluble or insoluble. Reacting with ammonia or ethanol, CaCl2·8NH3 and CaCl2·4C2H5OH complexes were formed, respectively. At low temperature, the solution crystallizes and precipitates as a hexahydrate, which is gradually dissolved in its own crystalline water when heated to 30 ° C, and gradually loses water when heated to 200 ° C, and becomes a dihydrate when heated to 260 ° C, which becomes a white porous anhydrous calcium chloride. Some hydrolysis reactions occur in the process of heating dehydration of hydrated calcium chloride, so the products often contain a small amount of CaO impurities.
Use 1: Used as refrigerant, also used in food processing, pharmaceutical and so on.
Use 2: Used in the food industry as calcium fortifying agent, chelating agent, curing agent and refrigerating refrigerant.
Use 3: for feed calcium supplement.
Use 4: As a coagulant, China stipulates that it can be used in soybean products according to production needs.
Use 5: used as a refrigerant (such as frozen brine for freezers, frozen brine for ice making and Popsicle making), antifreeze, automobile antifreeze, fire extinguishing agent. Flame retardant for melting ice and melting snow, finishing and finishing of cotton fabrics. Used as a adhesive and wood preservative. It is a raw material for the manufacture of anhydrous calcium chloride. Used in wall painting and plastering operations to increase condensation capacity. Rubber is produced for use as a condensate agent. Mix starch paste as gluing agent. It is also used for non-ferrous metal smelting. Used as medicine.
Use 6: oxygen, sulfur absorbent. Food protectant. Sizing agent. Water purification agent. Antifreeze.
我們擁有許多深度合作的優質工廠,可以為您提供高品質的產品和有競爭力的價格。並且我們還可以對大量採購給予折扣。確認付款後,交貨時間約 3-20 天。




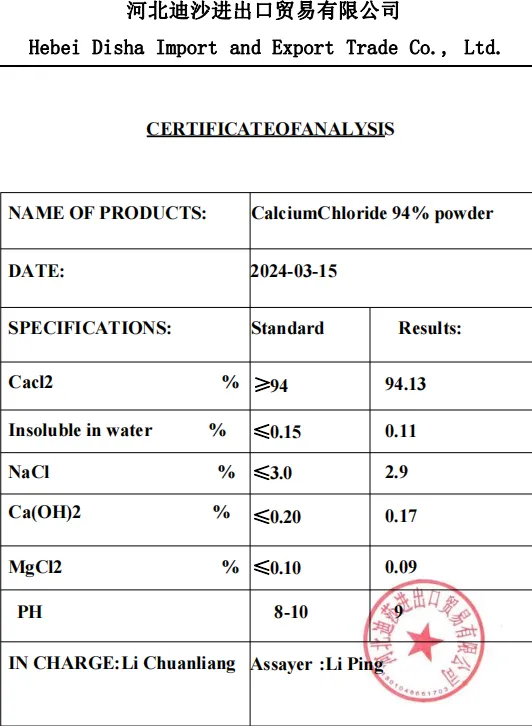
Electrolyte properties:
In an aqueous solution, calcium chloride fully ionizes into Ca² + and Cl⁻ ions, exhibiting the properties of a strong electrolyte. This electrolyte property makes calcium chloride solution with high conductivity and effective conduction of current, but also makes it an important chemical raw material and analytical reagent. In the fields of water treatment, metal processing and chemical synthesis, this property of calcium chloride can be used effectively for ion exchange, pH regulation and precipitation of metal ions.
Reactions with other chemicals:
Calcium chloride can also undergo a double substitution reaction with sodium sulfate (Na2SO4) and other salts to produce sodium chloride and calcium sulfate precipitation. The properties of this chemical reaction have important applications in industrial production and laboratory analysis, such as for the adjustment of water hardness or as a precipitating agent.
產品類別
-
 Apr . 27, 2025Zibo will host the 2025 International Chemical ExpoZibo, a city known for its thriving chemical industry, will host the 2025 Zibo International Chemical Expo from May 16 to May 18, 2025. This highly anticipated event aims to bring together industry leaders, innovators and stakeholders from around the world to explore the latest advancements and trends in the chemical industry.
Apr . 27, 2025Zibo will host the 2025 International Chemical ExpoZibo, a city known for its thriving chemical industry, will host the 2025 Zibo International Chemical Expo from May 16 to May 18, 2025. This highly anticipated event aims to bring together industry leaders, innovators and stakeholders from around the world to explore the latest advancements and trends in the chemical industry. -
 Apr . 22, 20252025 Yokohama Cosmetics Raw Materials and Technology ExhibitionYOKOHAMA, Japan – The City of Yokohama is preparing to host the much-anticipated Cosmetics Ingredients & Technologies 2025 from May 14 to May 16, 2025. The premier event is expected to attract industry professionals, innovators and enthusiasts from around the world to showcase the latest advancements in cosmetic ingredients and technologies.
Apr . 22, 20252025 Yokohama Cosmetics Raw Materials and Technology ExhibitionYOKOHAMA, Japan – The City of Yokohama is preparing to host the much-anticipated Cosmetics Ingredients & Technologies 2025 from May 14 to May 16, 2025. The premier event is expected to attract industry professionals, innovators and enthusiasts from around the world to showcase the latest advancements in cosmetic ingredients and technologies. -
 Apr . 18, 20252025 India Mumbai Fine Chemicals ExhibitionMUMBAI, India – The bustling metropolis of Mumbai is gearing up to host the much-anticipated Fine Chemicals Expo on April 29-30, 2025. The premier event is expected to attract industry leaders, innovators and stakeholders from across the world to showcase the latest advancements in the fine chemicals sector.
Apr . 18, 20252025 India Mumbai Fine Chemicals ExhibitionMUMBAI, India – The bustling metropolis of Mumbai is gearing up to host the much-anticipated Fine Chemicals Expo on April 29-30, 2025. The premier event is expected to attract industry leaders, innovators and stakeholders from across the world to showcase the latest advancements in the fine chemicals sector.













