Warning: Undefined array key "title" in /home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/1198/header.php on line 6
Warning: Undefined array key "file" in /home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/1198/header.php on line 7
Warning: Undefined array key "title" in /home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/1198/header.php on line 7
Warning: Undefined array key "title" in /home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/1198/header.php on line 7
- អាហ្រ្វិក
- អាល់បានី
- អាំហារិក
- ភាសាអារ៉ាប់
- អាមេនី
- អាស៊ែបៃហ្សង់
- បាស
- បេឡារុស្ស
- បង់ក្លាដែស
- បូស្នៀ
- ប៊ុលហ្គារី
- កាតាឡាន
- សេប៊ូណូ
- ចិន
- ចិន (តៃវ៉ាន់)
- Corsican
- ក្រូអាត
- ឆេក
- ដាណឺម៉ាក
- ហូឡង់
- ភាសាអង់គ្លេស
- អេស្ប៉ារ៉ាន់តូ
- អេស្តូនី
- ហ្វាំងឡង់
- បារាំង
- ហ្វ្រីសៀន
- ហ្គាលីសៀន
- ហ្សកហ្ស៊ី
- អាឡឺម៉ង់
- ក្រិក
- ហ្គូចារ៉ាទី
- ក្រេអូល ហៃទី
- ហូសា
- ហាវ៉ៃ
- ភាសាហេព្រើរ
- ទេ
- មៅ
- ហុងគ្រី
- អ៊ីស្លង់
- អ៊ីកបូ
- ឥណ្ឌូនេស៊ី
- អៀរឡង់
- អ៊ីតាលី
- ជប៉ុន
- ជ្វា
- កាណាដា
- កាហ្សាក់ស្ថាន
- ខ្មែរ
- រវ៉ាន់ដា
- កូរ៉េ
- ឃឺដ
- កៀហ្ស៊ីស៊ី
- ជំងឺរបេង
- ឡាតាំង
- ឡាតវី
- លីទុយអានី
- លុចសំបួរ
- ម៉ាសេដូនៀ
- ម៉ាល់ហ្គាស៊ី
- ម៉ាឡេ
- ម៉ាឡាយ៉ាឡា
- ម៉ាល់តា
- ម៉ៅរី
- ម៉ារ៉ាធី
- ម៉ុងហ្គោលី
- មីយ៉ាន់ម៉ា
- នេប៉ាល់
- ន័រវេស
- ន័រវេស
- អូស៊ីតាន់
- ប៉ាសតូ
- ពែរ្ស
- ប៉ូឡូញ
- ព័រទុយហ្គាល់
- ពុនចាប៊ី
- រ៉ូម៉ានី
- រុស្សី
- សាម័រ
- ស្កុតឡេក
- ស៊ែប៊ី
- ភាសាអង់គ្លេស
- សូណា
- ស៊ីនឌី
- ស៊ីនហាឡា
- ស្លូវ៉ាគី
- ស្លូវេនី
- សូម៉ាលី
- ភាសាអេស្ប៉ាញ
- ស៊ុនដា
- ស្វាហ៊ីលី
- ស៊ុយអែត
- តាកាឡុក
- តាជីក
- តាមីល
- តាតា
- តេលូហ្គូ
- ថៃ
- ទួរគី
- តួកមេន
- អ៊ុយក្រែន
- អ៊ូឌូ
- អ៊ុយហ្គួរ
- អ៊ូសបេក
- វៀតណាម
- វែល
- ជំនួយ
- យីឌីស
- យូរូបា
- ហ្សូលូ
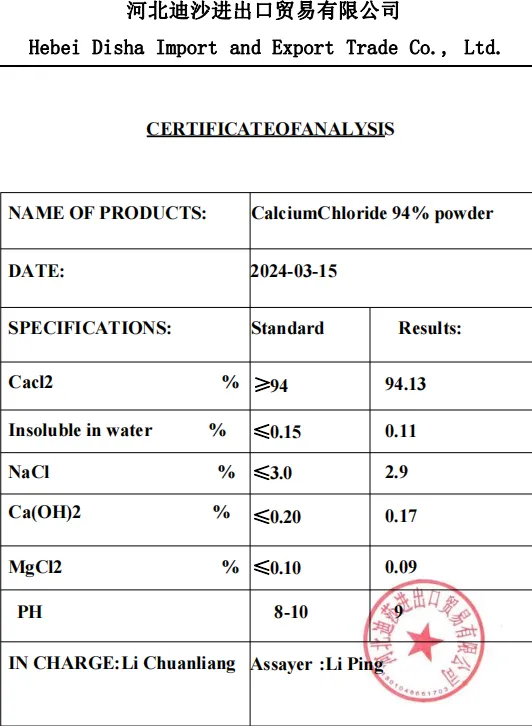
Calcium Chloride
Calcium chloride (chemical formula: CaCl2) is a white or yellowish solid inorganic compound, belonging to the salt class, is a typical ionic halide, because of its high solubility, moisture absorption and water removal and widely used in many fields. According to its hydrated form exists in different physical forms, the most common is dihydrate (CaCl2·2H2O), its high solubility allows it to dissolve quickly in water, releasing a large amount of heat, and is therefore very useful in applications requiring rapid heating or drying. In addition, calcium chloride is also commonly used in salt water, including road deicing agents and desiccant used in refrigeration equipment.




Colorless cubic crystal, white or off-white, granular, honeycomb block, spheroid, irregular granular, powdered. Slightly toxic, odorless, slightly bitter taste. It deliqueses easily when exposed to air. It is easily soluble in water, with a solubility of 74.0g /100 g water at 20 ° C, while releasing a large amount of heat (the dissolution enthalpy of calcium chloride is -176.2cal /g). This property makes it very useful in applications where rapid heat generation is required, such as as a heat source in certain heating processes, in addition, the specific heat capacity and thermal conductivity of calcium chloride are also important physical parameters that affect its efficiency in thermal management and regulatory applications. The calcium chloride solution is neutral. Soluble in a variety of polar, protic solvents, solubility at 20℃ in the following solvents (g/100mL solvent) : methanol: 29.2, anhydrous ethanol: 25.8, n-propanol: 15.8, n-butanol: 25.0, n-amyl alcohol: 11.5, ethylene glycol: 21.6 (25℃), formic acid: 43.1, acetic acid: 15.0 (30℃), hydrazine: 16.0. However, in dipolar solvents and low polar solvents, such as ether, tetrahydrofuran, etc., it is only slightly soluble or insoluble. Reacting with ammonia or ethanol, CaCl2·8NH3 and CaCl2·4C2H5OH complexes were formed, respectively. At low temperature, the solution crystallizes and precipitates as a hexahydrate, which is gradually dissolved in its own crystalline water when heated to 30 ° C, and gradually loses water when heated to 200 ° C, and becomes a dihydrate when heated to 260 ° C, which becomes a white porous anhydrous calcium chloride. Some hydrolysis reactions occur in the process of heating dehydration of hydrated calcium chloride, so the products often contain a small amount of CaO impurities.
Use 1: Used as refrigerant, also used in food processing, pharmaceutical and so on.
Use 2: Used in the food industry as calcium fortifying agent, chelating agent, curing agent and refrigerating refrigerant.
Use 3: for feed calcium supplement.
Use 4: As a coagulant, China stipulates that it can be used in soybean products according to production needs.
Use 5: used as a refrigerant (such as frozen brine for freezers, frozen brine for ice making and Popsicle making), antifreeze, automobile antifreeze, fire extinguishing agent. Flame retardant for melting ice and melting snow, finishing and finishing of cotton fabrics. Used as a adhesive and wood preservative. It is a raw material for the manufacture of anhydrous calcium chloride. Used in wall painting and plastering operations to increase condensation capacity. Rubber is produced for use as a condensate agent. Mix starch paste as gluing agent. It is also used for non-ferrous metal smelting. Used as medicine.
Use 6: oxygen, sulfur absorbent. Food protectant. Sizing agent. Water purification agent. Antifreeze.
យើងមានរោងចក្រដែលមានគុណភាពខ្ពស់ជាច្រើនជាមួយនឹងកិច្ចសហប្រតិបត្តិការយ៉ាងស៊ីជម្រៅ ដែលអាចផ្តល់ឱ្យអ្នកនូវផលិតផលដែលមានគុណភាពខ្ពស់ និងតម្លៃប្រកួតប្រជែង។ ហើយយើងក៏អាចផ្តល់ការបញ្ចុះតម្លៃសម្រាប់ការទិញច្រើនផងដែរ។ហើយយើងសហការជាមួយក្រុមហ៊ុនដឹកជញ្ជូនដែលមានជំនាញវិជ្ជាជីវៈជាច្រើន អាចចែកចាយផលិតផលដោយសុវត្ថិភាព និងរលូនដល់ដៃរបស់អ្នក។ ពេលវេលាដឹកជញ្ជូនគឺប្រហែល 3-20 ថ្ងៃបន្ទាប់ពីការបញ្ជាក់ការទូទាត់។




Electrolyte properties:
In an aqueous solution, calcium chloride fully ionizes into Ca² + and Cl⁻ ions, exhibiting the properties of a strong electrolyte. This electrolyte property makes calcium chloride solution with high conductivity and effective conduction of current, but also makes it an important chemical raw material and analytical reagent. In the fields of water treatment, metal processing and chemical synthesis, this property of calcium chloride can be used effectively for ion exchange, pH regulation and precipitation of metal ions.
Reactions with other chemicals:
Calcium chloride can also undergo a double substitution reaction with sodium sulfate (Na2SO4) and other salts to produce sodium chloride and calcium sulfate precipitation. The properties of this chemical reaction have important applications in industrial production and laboratory analysis, such as for the adjustment of water hardness or as a precipitating agent.
ប្រភេទផលិតផល
-
 Jun . 06, 2025Xanthan Gum Replacement and Powder InsightsReplacing xanthan gum, understanding xanthan gum gum properties, and selecting high-quality xanthan gum powder are critical considerations for food manufacturers and formulators.
Jun . 06, 2025Xanthan Gum Replacement and Powder InsightsReplacing xanthan gum, understanding xanthan gum gum properties, and selecting high-quality xanthan gum powder are critical considerations for food manufacturers and formulators. -
 Jun . 06, 2025Exploring SLES 70 in DepthSles 70, sodium lauryl ether sulfate 70, and sodium lauryl sulfate 70 are among the most widely used surfactants in personal care and cleaning product formulations.
Jun . 06, 2025Exploring SLES 70 in DepthSles 70, sodium lauryl ether sulfate 70, and sodium lauryl sulfate 70 are among the most widely used surfactants in personal care and cleaning product formulations. -
 Jun . 06, 2025E1520 Propylene Glycol Uses and Consumption PatternsE1520 propylene glycol, commonly known as propylene glycol, is a versatile chemical compound with extensive applications across multiple industries.
Jun . 06, 2025E1520 Propylene Glycol Uses and Consumption PatternsE1520 propylene glycol, commonly known as propylene glycol, is a versatile chemical compound with extensive applications across multiple industries.













