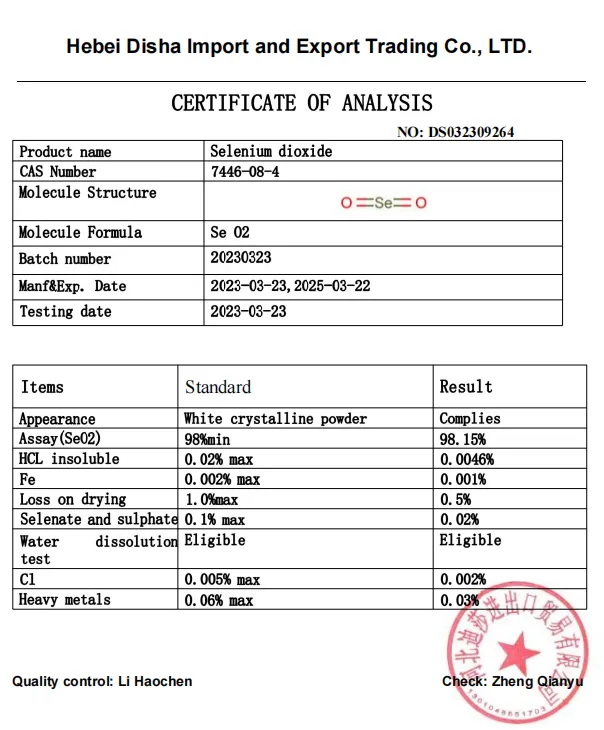
Selenium Dioxide
Selenium dioxide is a kind of oxide, chemical formula SeO2, white crystalline powder, vapor is yellow green, soluble in water and polar organic solvents, used as organic compound oxidant, catalyst, chemical reagent, all kinds of inorganic selenium compounds manufacturing raw materials.




Appearance: white crystalline powder.
Solubility: Soluble in water and polar organic solvents.
1. It is used to produce high-purity selenium and other selenium compounds, and also serves as an oxidizing agent and catalyst for organic synthesis of drugs.
2. It is also used as a special reagent for plant alkaloids, and can be used to precipitate zirconium, hafnium, and prepare selenium compounds.
3. It is used as an oxidizing agent, catalyst, and chemical reagent for organic compounds, as well as a raw material for the manufacture of various inorganic selenium compounds. It is also used in copiers, rectifiers, etc.
4. It is used to manufacture other selenium compounds, to determine plant alkaloids, as an oxidizing agent. When reacted with ammonia, it produces nitrogen and selenium, and when reacted in an alcohol solution, it produces ethylammonium selenite [(NH4)2C2H5SeO3]. When reacted with hydrazine, it produces nitrogen and a black amorphous selenium, and when reacted with hydroxylamine hydrochloride, it produces nitrogen and a red-brown amorphous selenium, and when reacted with nitric acid, it produces monoselenous acid.
A ni ọpọlọpọ awọn ile-iṣelọpọ giga-giga pẹlu ifowosowopo jinlẹ, eyiti o le fun ọ ni awọn ọja to gaju ati awọn idiyele ifigagbaga. Ati pe a tun le fun awọn ẹdinwo fun awọn rira olopobobo.Ati pe a ṣe ifọwọsowọpọ pẹlu ọpọlọpọ awọn ile-iṣẹ gbigbe ẹru ẹru ọjọgbọn, le fi awọn ọja ranṣẹ lailewu ati laisiyonu si ọwọ rẹ. Akoko ifijiṣẹ jẹ nipa awọn ọjọ 3-20 lẹhin ijẹrisi isanwo.




1. It is a white, light-sensitive, crystalline powder with a sharp, acidic odor and a burning sensation. Its vapor is yellow-green in color and has a pungent taste. It is stable to light and heat, has hygroscopicity, and has a pungent taste. It has oxidizing properties and will precipitate elemental selenium from organic reducing agents. It is toxic.
2. It is easily reduced to selenium.
3. Use protective equipment when handling. Pay attention to regularly checking the concentration of selenium in the air. Regularly check the body. Provide a diet rich in protein and amino acids.
4. It is stable to air and moisture. Due to its toxicity, it is recommended to handle and use with caution in a fume hood.
Awọn ẹka ọja
















