Warning: Undefined array key "file" in /home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/1198/header.php on line 7
Warning: Undefined array key "title" in /home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/1198/header.php on line 7
Warning: Undefined array key "title" in /home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/1198/header.php on line 7
- Afrikaly
- Alban
- Amhar
- Arapça
- Ermeni
- Azerbaýjan
- Bask
- Belarus
- Bengali
- Bosniýa
- Bolgar
- Katalan
- Sebuano
- Hytaý
- Hytaý (Taýwan)
- Korsikan
- Horwatiýa
- Çeh
- Daniýaly
- Gollandiýaly
- Iňlis
- Esperanto
- Eston
- Fin
- Fransuz
- Frizian
- Galisiýa
- Gürji
- Nemes
- Grek
- Gujarati
- Gaiti kreoly
- hausa
- hawaiian
- Hebrewewreýçe
- .Ok
- Miao
- Wenger
- Islandiýa
- igbo
- Indoneziýaly
- irish
- Italýan
- Japaneseaponlar
- Javaneseawan
- Kannada
- Gazak
- Khmer
- Ruanda
- Koreýçe
- Kürt
- Gyrgyzystan
- Inçekesel
- Latyn
- Latwiýa
- Litwa
- Lýuksemburg
- Makedoniýa
- Malgaşi
- Malaý
- Malaýalam
- Malta
- Maori
- Marathi
- Mongol
- Mýanma
- Nepali
- Norweg
- Norweg
- Oksitan
- Puştun
- Pars
- Polýak
- Portugaliýa
- Penjabi
- Rumyn
- Rus
- Samoan
- Şotlandiýaly Gael
- Serb
- Iňlis
- Şona
- Sindhi
- Sinhala
- Slowakiýa
- Sloweniýa
- Somali
- Ispan
- Sundanese
- Suwaýili
- Şwesiýa
- Tagalog
- Täjik
- Tamil
- Tatar
- Telugu
- Taý
- Türk
- Türkmenler
- Ukrain
- Urdu
- Uýgur
- Özbek
- Wýetnamly
- Uels
- Kömek ediň
- Yiddishahudy
- Yorubaoruba
- Zulu
Tirzepatide CAS 2023788
Tirzepatide (LY3298176) is a glucose-dependent insulin nutritive polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor dual agonist being developed for the treatment of type 2 diabetes. Tirzepatide (LY3298176) was significantly more effective in blood glucose control and weight loss than duralutide.


First, Tirzepatide is a human glucagon-like peptide-1 (GLP-1) receptor agonist that reduces blood sugar levels by binding to GLP-1 receptors in the human body, stimulating insulin secretion and inhibiting glucagon secretion. Compared with traditional diabetes drugs, the mechanism of action of Tirzepatide is more in line with the physiological state of the human body and can better control blood sugar levels.
Secondly, the efficacy of Tirzepatide is significant. According to the results of multiple clinical trials, patients treated with Tirzepatide show significant efficacy in reducing blood sugar levels, while also effectively reducing weight and blood pressure. These effects have important implications for protecting the cardiovascular health of patients.
In addition, Tirzepatide has relatively few side effects. Although the drug may also cause some adverse reactions, such as nausea, vomiting, diarrhea, etc., these symptoms are usually mild and can be tolerated by patients. Tirzepatide has a lower incidence of side effects and a higher safety profile than traditional diabetes drugs.
Finally, Tirzepatide is easy to use. The drug can be used either alone or in combination with other diabetes medications. Patients only need to give the drug by subcutaneous injection, easy to operate, convenient to carry and use.
In summary, Tirzepatide, as a new type of diabetes treatment drug, has the advantages of significantly reducing blood sugar level, fewer side effects and convenient use. Tirzepatide is a good choice for patients who need to control their blood sugar levels for a long time.
Tirzepatide is a once-weekly injection of GIP and GLP-1 receptor agonist, which is a single molecule that activates the body's two natural incretin receptors, GIP and GLP-1. GIP is a hormone that complements the action of GLP-1 receptor agonists.
In preclinical models, GIP has been shown to reduce food intake and increase energy expenditure resulting in lower body weight, and when combined with GLP-1 receptor agonists, may have a greater impact on markers of metabolic disorders such as body weight, blood sugar, and lipids.
Tirzepatide has been approved in the United States, the United Arab Emirates and the European Union for improving blood sugar control in adults with type 2 diabetes based on diet control and exercise.
The factories we cooperate with have large shipments of Tirzepatide, rapid delivery and fresh production dates. This can help some suppliers and buyers to buy high-quality Tirzepatide with fresh production date from us. We will not sell products near the expiration date to customers like others, because transportation takes time, so our delivery cycle is usually arranged in 10-15 days, and orders under 10 tons will be shipped within 10 days.




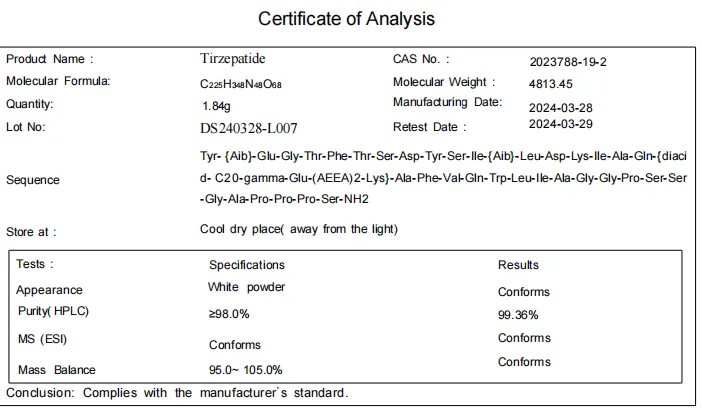
Recommended dosage and method of administration:
The recommended starting dose of Tirzepatide is 2.5 mg once a week by subcutaneous injection. The 2.5 mg dose is used at the beginning of treatment and is not used for blood glucose control. After 4 weeks, the dose was increased to 5 mg and injected subcutaneously once a week.
If additional blood sugar control is needed, increase the dose in 2.5 mg increments at least 4 weeks after the current dose.
The maximum dose of Tirzepatide is 15 mg, given subcutaneously once a week.
If a dose is missed, instruct the patient to receive Tipotide as soon as possible within 4 days (96 hours) of the missed dose. If it is more than 4 days, skip the missed dose and use the next dose on a regularly scheduled date, after which the original regular weekly dosing schedule is resumed.
If necessary, the weekly dosing date can be changed, as long as the time interval between dosing is at least 3 days (72 hours).
Medication precautions:
Use Tirzepatide once a week, with or without meals at any time of the day.
Tirzepatide is injected subcutaneously in the abdomen, thigh or upper arm.
Rotate the injection site with each injection.
Visual examination of Tirzepatide before injection should be clear, colorless to yellowish. Do not use if you see particulate matter or discoloration.
When Tirzepatide is used with insulin, it should be injected separately and never mixed. Tirzepatide and insulin can be injected in the same part of the body, but not too close together.
Önüm kategoriýalary
-
 May . 07, 20252025 New York Cosmetics Ingredients ExhibitionThe much-anticipated 2025 Cosmetics Ingredients New York will be held at the Javits Center in New York from June 3 to 4, 2025. This event will bring together industry leaders, innovators and enthusiasts from all over the world to discuss the latest trends and advances in the field of cosmetic ingredients.
May . 07, 20252025 New York Cosmetics Ingredients ExhibitionThe much-anticipated 2025 Cosmetics Ingredients New York will be held at the Javits Center in New York from June 3 to 4, 2025. This event will bring together industry leaders, innovators and enthusiasts from all over the world to discuss the latest trends and advances in the field of cosmetic ingredients. -
 Apr . 27, 2025Zibo will host the 2025 International Chemical ExpoZibo, a city known for its thriving chemical industry, will host the 2025 Zibo International Chemical Expo from May 16 to May 18, 2025. This highly anticipated event aims to bring together industry leaders, innovators and stakeholders from around the world to explore the latest advancements and trends in the chemical industry.
Apr . 27, 2025Zibo will host the 2025 International Chemical ExpoZibo, a city known for its thriving chemical industry, will host the 2025 Zibo International Chemical Expo from May 16 to May 18, 2025. This highly anticipated event aims to bring together industry leaders, innovators and stakeholders from around the world to explore the latest advancements and trends in the chemical industry. -
 Apr . 22, 20252025 Yokohama Cosmetics Raw Materials and Technology ExhibitionYOKOHAMA, Japan – The City of Yokohama is preparing to host the much-anticipated Cosmetics Ingredients & Technologies 2025 from May 14 to May 16, 2025. The premier event is expected to attract industry professionals, innovators and enthusiasts from around the world to showcase the latest advancements in cosmetic ingredients and technologies.
Apr . 22, 20252025 Yokohama Cosmetics Raw Materials and Technology ExhibitionYOKOHAMA, Japan – The City of Yokohama is preparing to host the much-anticipated Cosmetics Ingredients & Technologies 2025 from May 14 to May 16, 2025. The premier event is expected to attract industry professionals, innovators and enthusiasts from around the world to showcase the latest advancements in cosmetic ingredients and technologies.













