Warning: Undefined array key "title" in /home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/1198/header.php on line 6
Warning: Undefined array key "file" in /home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/1198/header.php on line 7
Warning: Undefined array key "title" in /home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/1198/header.php on line 7
Warning: Undefined array key "title" in /home/www/wwwroot/HTML/www.exportstart.com/wp-content/themes/1198/header.php on line 7
Hebei Yize Trade Center Co., LTD.!
Φεβ . 17, 2025 11:23 Back to list
aspartame how is it made
Aspartame, a low-calorie sweetener widely used in food and beverage products, has garnered attention both for its sweetness and the fascinating process by which it is made. Understanding the production of aspartame offers insights into its unique properties and helps dispel myths surrounding its use.
This synthesis process ensures that the resulting aspartame is of high purity, usually exceeding 98%. However, this isn't the end. Quality control is paramount, with sophisticated chromatography and mass spectrometry used to ensure the product meets rigorous safety and purity standards before it hits the market. The creation of aspartame encapsulates an intriguing blend of organic chemistry and biotechnology. It's the result of years of research that achieved the synthesis of a sweetener closely mimicking the taste of sugar but without the same caloric density. Aspartame's production is a testament to the abilities of research and development teams to create safe and effective alternatives to traditional sweeteners. Aspartame production is also a reflection of the global arms of the food industry, connecting agriculture, chemistry, and consumer goods in a seamless chain. Each batch undergoes stringent safety assessments, to not only comply with food safety regulations globally but also to fit burgeoning consumer demands for low-calorie sweetening options without sacrificing taste or enjoyment. Aspartame, once demonized for its synthetic origins, is now a staple in products worldwide. Its creation relies on precise scientific processes, shared expertise across disciplines, and reflects an industry committed to innovation and consumer safety. Understanding its production not only underscores its safety but also enhances appreciation for the complexity behind what may seem a simple table-top condiment. In essence, aspartame's journey from laboratory to table exemplifies a larger narrative in food production technology and science harmonizing to provide solutions to contemporary consumer needs. It is a story marked by meticulous research, regulatory vigilance, and unwavering commitment to providing a product that satisfies both curiosity and culinary desires.
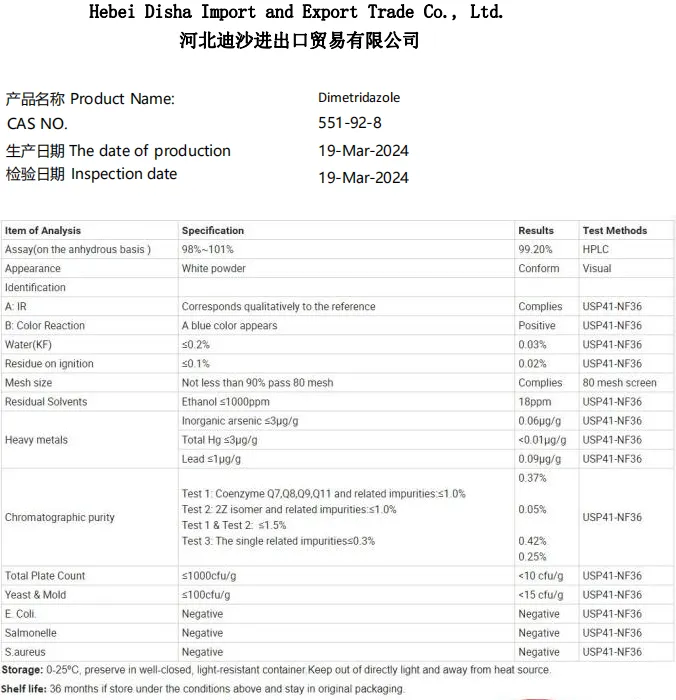

This synthesis process ensures that the resulting aspartame is of high purity, usually exceeding 98%. However, this isn't the end. Quality control is paramount, with sophisticated chromatography and mass spectrometry used to ensure the product meets rigorous safety and purity standards before it hits the market. The creation of aspartame encapsulates an intriguing blend of organic chemistry and biotechnology. It's the result of years of research that achieved the synthesis of a sweetener closely mimicking the taste of sugar but without the same caloric density. Aspartame's production is a testament to the abilities of research and development teams to create safe and effective alternatives to traditional sweeteners. Aspartame production is also a reflection of the global arms of the food industry, connecting agriculture, chemistry, and consumer goods in a seamless chain. Each batch undergoes stringent safety assessments, to not only comply with food safety regulations globally but also to fit burgeoning consumer demands for low-calorie sweetening options without sacrificing taste or enjoyment. Aspartame, once demonized for its synthetic origins, is now a staple in products worldwide. Its creation relies on precise scientific processes, shared expertise across disciplines, and reflects an industry committed to innovation and consumer safety. Understanding its production not only underscores its safety but also enhances appreciation for the complexity behind what may seem a simple table-top condiment. In essence, aspartame's journey from laboratory to table exemplifies a larger narrative in food production technology and science harmonizing to provide solutions to contemporary consumer needs. It is a story marked by meticulous research, regulatory vigilance, and unwavering commitment to providing a product that satisfies both curiosity and culinary desires.
Next:

